Department reviewer resources
The resources below are intended for those who serve in the department reviewer role. Department review is the step in the IRB process that comes after the principal investigator (PI) submits the study, but before the study is routed to the IRB for review.
Click through the accordions below to learn more about department review. The “Resources for Department Reviewers” section contains guides and tools to assist department reviewers in conducting department reviews.
Department review is required for all expedited and full board studies submitted to the IRB. Exempt studies do not require department review, though PIs are instructed to select a department reviewer when submitting an exempt study, in the event that the study is upgraded to expedited for full board review. If that occurs, the study will be routed for department review prior to returning to the IRB.
Department review is the step that comes after the PI submits the study, but before the study is routed to the IRB review.
The purpose of department review is twofold. First, it is for the department reviewer to review aspects of a study that impact participant rights and welfare, but which are outside the scope or expertise of the IRB. For example, reviewing for scientific merit requires someone familiar with the specific discipline, and scientific merit can impact the risk/benefit calculation for a study (i.e., it is not worth exposing participants to risk for a non-meritorious study). Likewise, departmental resources can also impact the potential success of the research, thus impacting the risk/benefit ratio of the research, but the IRB has no way of knowing the details of resources within a given department.
Second, department review provides departments with the opportunity to become aware of and track the research going on in their departments. For example, tracking space and equipment usage, tracking the proportion of student-initiated studies, or tracking sources of funding may be of importance or may be useful to departments.
A department reviewer should be external to the study. By default, the department chair should serve as the department reviewer, but if the department chair is involved in the study, then the next choice for department reviewer should be the applicable dean. Department chairs and deans are allowed to designate another individual as department reviewer, but they should follow these guidelines when selecting a designee:
- Ideally, the department reviewer should not be subordinate to the PI
- Ideally, the department reviewer also conducts research, meaning they would be familiar with the discipline’s best practices for scientific design, and the relevant literature in the discipline
- The department reviewer should be familiar with the discipline and the department’s resources to make determinations relating to scientific merit and the adequacy of departmental resources
- A department research administrator is not a good choice for a department reviewer, since they may not have enough knowledge of the discipline to review for scientific merit
A department reviewer reviews for:
- PI qualifications
- Adequate resources
- Scientific merit
A common delay in IRB review relates to the designation of the project’s PI, so department reviewers are asked to confirm that a PI is eligible to serve in the role of PI, and has enough training/experience to conduct the research ethically and effectively.
Resource considerations include things like space/equipment, financial resources, and personnel. For example, a study working with Limited English Proficiency individuals might require a translator/interpreter to be on staff.
Reviewing for scientific merit helps assure the IRB that the studies it reviews are designed appropriately, conform to discipline best practices, and have a reasonable chance of answering the research questions or achieving the research aims.
Resources for department reviewers
- Department head reviews are now completed in the VIRBs as ancillary reviews.
- Department head review must occur prior to submission to the IRB for expedited and full board reviews.
Department head, or designee, review and approval for new studies will still take place in VIRBs. Department head approval is required for:
- All new submissions: excludes NHSR and exemption determination requests
- Submissions requesting reliance on an external IRB
- Submissions involving Humanitarian Use Device(s)
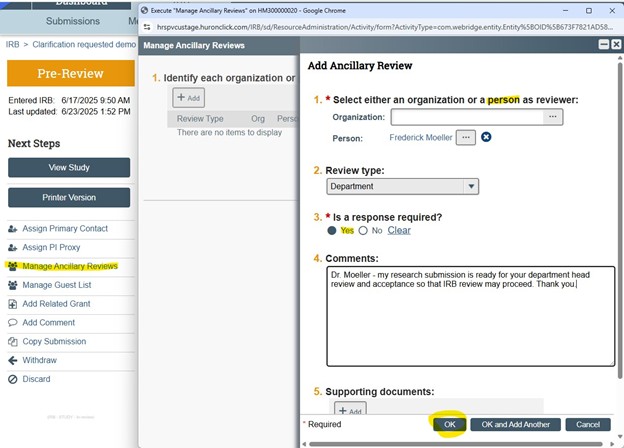
To initiate a request for department head approval for new submissions:
- Create new study in VIRBs
- Select the “Manage Ancillary Reviews” activity
- Request review by a Person; Review Type = Department; Response IS required; Enter Comments to communicate review request to the person identified; click OK
- Upon submission to the IRB the department head will be notified; the review must be accepted before IRB review may commence – the IRB may put the study in Clarifications Requested to reflect the pending department head review state
(Text description for image above)
- Department reviewer checklist (VCU eID is required to access). Use this checklist to guide your department review and document your findings.
The VCU IRB offers guidance and training to those who serve in the department reviewer role. To learn more, contact the education and outreach manager at irbeducation@vcu.edu.
Contact us
Please use the following contact information for:
- Reporting for single patient access and emergency uses of FDA regulated products, or
- General questions and questions related to IRB submissions

Questions related to single IRB, reliance agreements and/or external IRBs:

For individual staff email addresses, please see Contact us page for staff directory