The Investigator Manual is designed to guide you through policies and procedures related to the conduct of Human Research that are specific to this institution. The manual is comprehensive and covers everything from who can serve as PI and the IRB review process, to investigator responsibilities post-approval.
The New IRB system (VIRBs) web page has all guidance and training related to the VIRBs.
You need IRB review if your activity meets the regulatory definitions of "research involving a human subject,". This means it must involve both "research" and "human subjects". Refer to HRP-310 - WORKSHEET - Human research determination to help you determine if an activity meets the criteria.
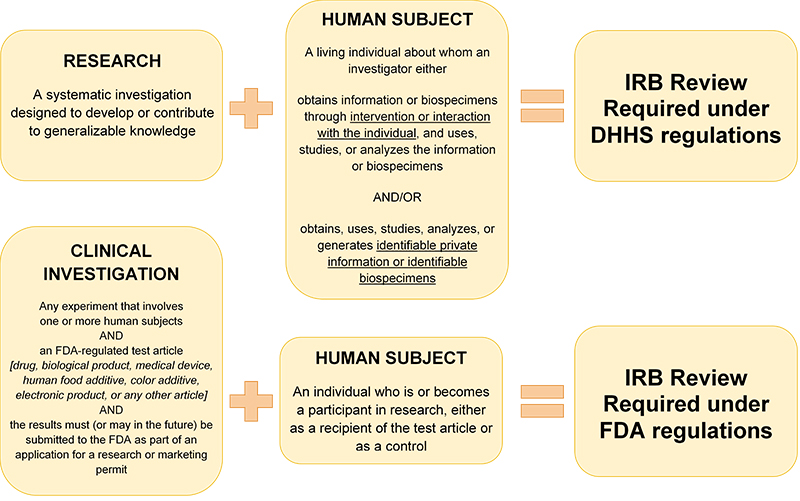
You can learn more about what these definitions mean and what activities are and are not subject to IRB review by visiting our “How do I know if I need IRB review?” page.
VCU fulfills the federal mandate to ensure all researchers involved in human subjects research are trained by requiring initial and continuing education through the Collaborative IRB Training Initiative (CITI).
All VCU personnel engaged in research must have completed the following VCU-specific CITI training through their VCU-affiliated CITI accounts:
- Human subjects protection
- Good Clinical Practice (GCP) - only required if your activity meets the NIH definition of a clinical trial.
VIRBs will link your CITI certificates with your study application. This means you are no longer required to upload your certificates to your study. To make sure your certificates link correctly, your CITI email must be the same email you use for VIRBs.
Learn more about required initial training and continuing education requirements on our CITI requirements webpage.
Complete COI disclosures
Prior to submitting expedited or full board projects for IRB review, designated COI investigators must complete any required COI disclosures. The COI review of those disclosures may occur concurrently with IRB review, but IRB approval cannot occur until it is determined that no conflicts exist or any conflicts have been acceptably managed.
To learn more about COI requirements, COI investigators and financial disclosures, you can visit our COI guidance page and/or the conflict of interest website.
Do I need any other ancillary reviews?
There are several ancillary reviews and processes required by VCU and VCUHS. Ancillary reviews are reviews by other VCU compliance groups or individuals that inform the IRB’s review of a new study or a modification to an existing study.
A summary of the ancillary reviews, including websites and contact details, can be found in HRP-309 - WORKSHEET - Ancillary review matrix.
The impact of an ancillary review group’s approval on the IRB’s review process varies.
VIRBs (VCU IRB system)
VCU uses an electronic protocol management system to manage IRB review. This system is called VIRBs (VCU IRB system). All information pertaining to VIRBs implementation can be found on the New IRB review system: VIRBs (VCU IRB system) page.
You can access VIRBs by clicking the button below.
It is important to note that if you are off campus (or using hospital network computers), you may need to be connected to the VCU VPN to access VIRBs. You can learn more about the VPN by visiting the Ask IT website for the VPN.
Access our researcher-focused guidance on the VCU HRPP Kaltura channel.
Click on the links below to access resources that will help you learn to navigate VIRBs:
- VIRBs training (single-site): IRB 10.5 single site on Vimeo (Huron)
- Researcher's guide (pdf)
- VIRBs training (multi-site): IRB 10.5 multi-site on Vimeo (Huron)
- Multi-site guide (pdf)
- *All training videos can be found on the HRPP Kaltura channel. Dual authentication sign-on is required for access to videos in Kaltura.
To learn more about what to include in your initial submission to the IRB, visit our Type of reviews webpage.
Single IRB review/IRB reliance
Investigators who wish to rely on an external IRB, or who are conducting research for which non-VCU sites will be relying on the VCU IRB, should visit our IRB reliance website for more information on single IRB review/IRB reliance.
Templates
Researchers should use the HRP-500 templates developed by the HRPP to build their submission in VIRBs. All updated templates can be found in the VIRBs library feature, on the HRPP policies and guidance web page and on the HRPP/IRB section of OVPRI’s forms page.
Additional templates, including short form consents, can be found in the HRPP/IRB section of OVPRI’s forms page.
Once the IRB submission is completed, the principal investigator or their "PI proxy" should submit the study for IRB review.
The following documents must be uploaded to your application:
- Study protocol
- PI C.V.
Examples of documents that must also be uploaded to if they are applicable to your project are:
- Consent and assent forms
- HIPAA form
- Investigator brochure
- Device manuals
- Recruitment materials
- Study measures (e.g. questionnaires and surveys)
Other documents may be required depending on your projects’ activities.
IRB oversight doesn't end after obtaining approval for an initial submission. There are other submissions to the IRB that happen during the life of a study, such as problem reporting, amendments, continuing reviews and closure requests. Please refer to the “What are my obligations after IRB approval?” in HRP-103 - Investigator manual for a complete list of your ongoing responsibilities.
You can stay in touch with the IRB by following our blog. Visit the blog to learn about upcoming events, training/education resources and receive emails with other important news and announcements.
When an expedited or full board study meets the criteria for closure, IRB oversight is no longer needed, and a study may be closed with the IRB.
To close a study the following criteria must be met:
- Study is permanently closed to enrollment OR was never open for enrollment
- All subjects have completed all study-related interventions OR not applicable
- Collection of private identifiable information is complete OR not applicable
- Analysis of private information is complete
Closure applications can be submitted via VIRBs using the "continuing review/study closure" function.
Studies that close with the IRB are still subject to data retention and access policies for VCU. You can learn more by visiting the VCU research data ownership, retention and access policy.
Research materials and data should be maintained and destroyed in accordance with VCU’s records management policies.
Contact us
Please use the following contact information for:
- Reporting for single patient access and emergency uses of FDA regulated products, or
- General questions and questions related to IRB submissions

Questions related to single IRB, reliance agreements and/or external IRBs:

For individual staff email addresses, please see Contact us page for staff directory