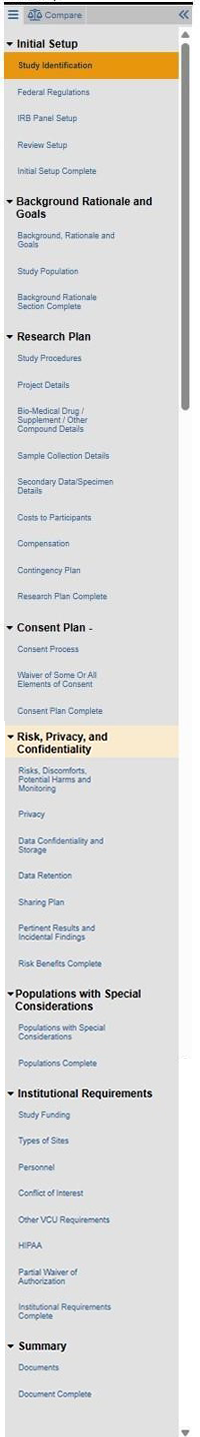
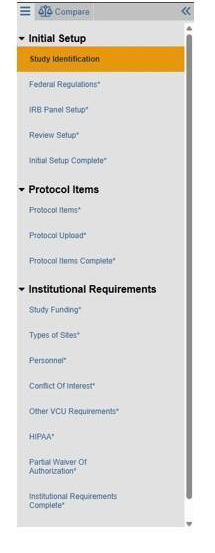
VCU’s Human Research Protection Program (HRPP) transitioned to a new IRB submission system on August 7, 2025. The new system is called:
VCU IRB system
VIRBs opened for submissions on August 7.
Most active non-exempt research undergoing continuing review and still enrolling participants will be required to transition to a protocol template (if a protocol is not already included in the submission) at some point in the coming year. Review “first touch options” below for study-specific considerations and flexible, PI-driven options for this transition timeline.
This page has all guidance and training related to the VIRBs.
Institutional reviews ancillary to IRB review and approval requirements may be referenced in HRP-309 - Ancillary review matrix. For all reviews required prior to or parallel with IRB review, upload the approval letter or confirmation of review under Local Site Documents as Other Attachments; e.g., PROC/PRMC, Informatics, Radiation Safety Committee, Institutional Biosafety Committee.
DMS plan and COI review will continue to take place in their current systems (Data Management System (DMS) and Activity and Interest Reporting System (AIRS), respectively).
The HRPP would like to provide the following clarification concerning the linking of DMS applications and IRB protocols:
- Please disregard the previously posted notice indicating the DMS would no longer be linked in VIRBs.
- The DMS application and IRB submissions will remain linked by HM# between the two application systems. When submitting a data management plan to DMS for review, follow the instructions posted on the DMS website for linking the DMP to the IRB application.
- To note: All institutional resources will be updated over time as the HRPP implements the VIRBs. Any current guidance that references to the archived IRB system, RAMS-IRB, will be reviewed and rolled out on an ongoing basis.
PIs will still need to upload any COI management plans to the applicable IRB submission in VIRBs, as indicated.
Department head, or designee, review and approval for new studies will still take place in VIRBs. Department head approval is required for:
- All new submissions: excludes NHSR and exemption determination requests
- Submissions requesting reliance on an external IRB
- Submissions involving Humanitarian Use Device(s)
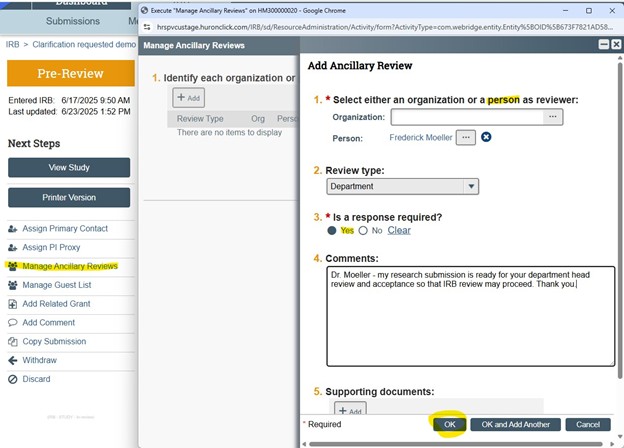
To initiate a request for department head approval for new submissions:
- Create new study in VIRBs
- Select the “Manage Ancillary Reviews” activity
- Request review by a Person; Review Type = Department; Response IS required; Enter Comments to communicate review request to the person identified; click OK
- Upon submission to the IRB the department head will be notified; the review must be accepted before IRB review may commence – the IRB may put the study in Clarifications Requested to reflect the pending department head review state
The HRPP is not currently planning to modify requirements around study team listing in VIRBs. The previous approach in RAMS IRB, consistent with prior HRPP guidance, to list COI Investigators including essential personnel remains acceptable.
Please note that Protocol Editors must be listed as study team members in VIRBs and will be designated under a new label: PI Proxy. Editors will be converted in the new system for existing studies.
VIRBs study team listing
Requirements and responsibilities
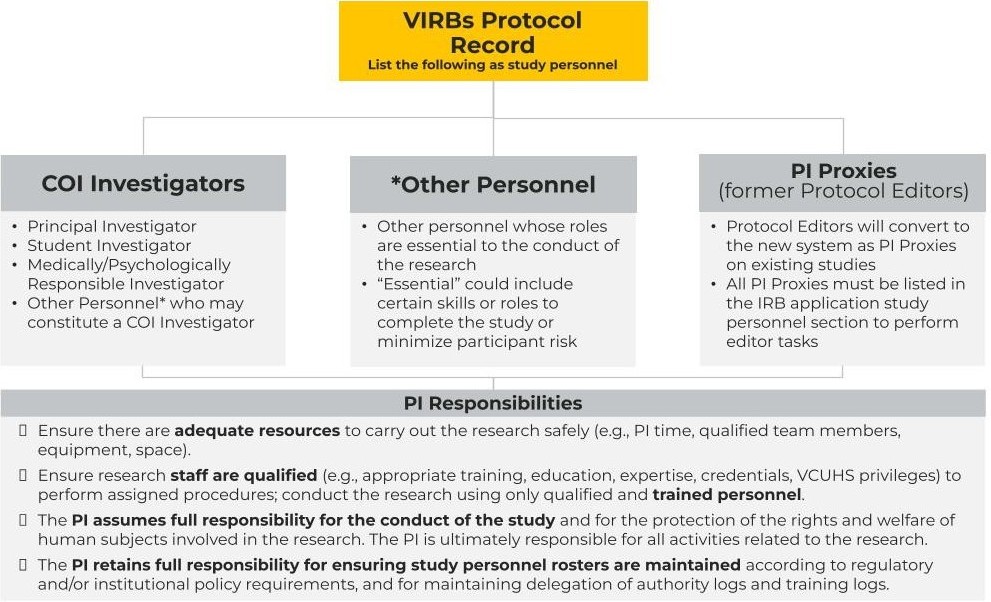
(Text description for image above)
Please reference the VCU Conflicts Of Interest in Research policy for additional information about COI Investigators.
Q: How do I know if my RAMS IRB submission did NOT convert to VIRBs effective 8/7/25?
A: The HM# assigned in RAMS IRB will convert to VIRBs and will be available for reference on the IRB workspace, active tab. You may search by ID (HM#), PI last name, name (of study), etc. If the study is not pulling up in VIRBs, check the status in RAMS IRB (in read only state after 8/1/25). Any project or follow-on submission not in a final state (e.g., approved, exempt, approved by external IRB) would not have converted to VIRBs and will need to be newly submitted.
Department head ancillary reviews in VIRBs
IRB community research system overview training
IRB research community initial single site submission training
IRB community research MOD, CR, MODCR, submission training
VIRBs training (single-site): IRB 10.5 single site on Vimeo (Huron)
Researcher's guide (pdf)
VIRBs training (multi-site): IRB 10.5 multi-site on Vimeo (Huron)
Multi-site guide (pdf)
*All training videos can be found on the HRPP Kaltura channel. Dual authentication sign-on is required for access to videos in Kaltura.
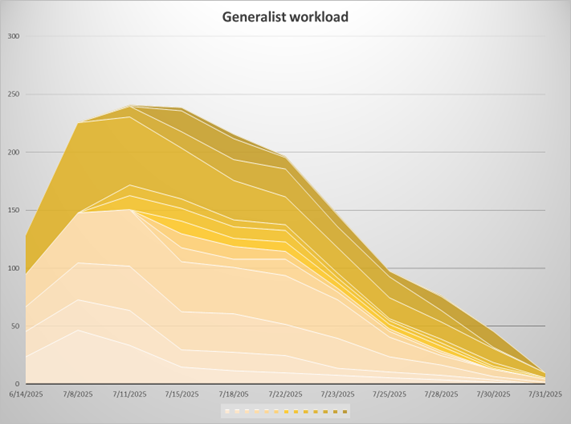
Thank you for your patience and support as we transition to the new VIRBs system. We were able to finalize most of our studies. This graph shows the mountain of applications processed by staff as RAMS-IRB was closed out.
Figure 1:
We are migrating 96% of all protocols from RAMS IRB into VIRBs (figure 2). That is 2,814 individual studies.
Figure 2:
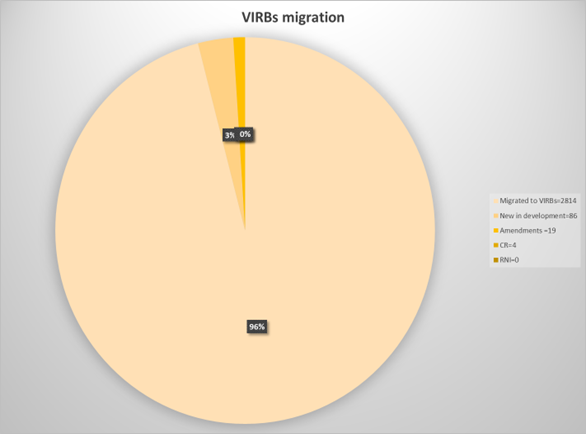
We know many of you submitted applications in the final weeks of RAMS IRB, and we want to ensure a smooth process for these remaining 4% studies.
The good news is, we have a clear plan for you.
What happens to your study submission
The short answer is: You will reload your submission documents into VIRBs after August 7, 2025. You will maintain read-only access to RAMS IRB, so you won't lose any of your previous work.
How to reload your application
In general, you'll simply start a new application in VIRBs, complete a short smart-form, and re-upload your submission documents. Ancillary reviews that were already completed do not need to be redone, though you may need a new departmental approval.
Instructions for specific application types
- New study applications: Start a new application in VIRBs. You'll be assigned a new HM#.
- To alert our staff, please email HRPP@vcu.edu with the subject line "Study reloaded in VIRBs" and provide the PI name, old HM#, and new HM#.
- Complete a short smart-form, and re-upload your study documents, addressing any previous comments.
- Log a comment in the new application: “Study reloaded in VIRBs: Old HM#”
- Amendments: Your parent study will be automatically moved to VIRBs (HM# unchanged). Start a new modification application in VIRBs and add a note in the "summarize the modifications" section stating: "This modification/amendment was submitted but not finalized in RAMS IRB."
- Complete a short smart-form, and re-upload your study documents, addressing any previous comments.
- Continuing reviews: Your study will transition to VIRBs if it has not expired. Please resubmit your continuing review application in the new system and address any comments that were left in RAMS IRB.
- Reliance studies: If your application was not finalized, it did not transition. Please reload your documents in VIRBs after August 7th.
- If you had completed "Step 1" in RAMS IRB, the study will quickly move to the “pending SIRB review” stage in VIRBs.
- If your application had pending comments, please address them and reload the updated documents to VIRBs.
We hope this clarifies the process. Please don't hesitate to reach out if you have further questions by emailing HRPP@vcu.edu.
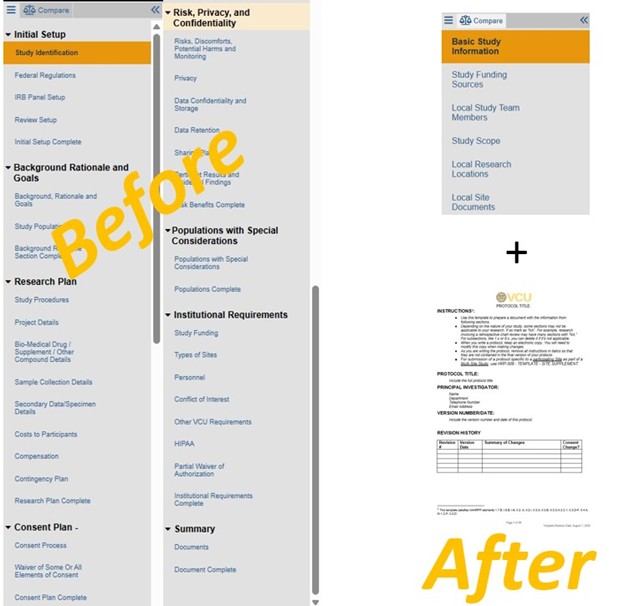
For most submissions that entered RAMS IRB as new projects before the 7/14/23 compliance date for use of protocol templates: a transition to protocol-based applications will be required.
This will apply to studies with an extended RAMS IRB smartform application and no stand-alone protocol template (HRP-503 / 503a, or a sponsor protocol with HRP-508 - Site Supplement, or another approved protocol template). See comparison of smartform application fields further below.
The HRPP has implemented a flexible transition approach so that study teams may pursue timing most conducive to each respective study.
- Transition before go-live by opening an Amendment: select the option to “Convert to Protocol Template” and click “OK” to permanently convert to the reduced smartform template. Use Protocol Upload to attach HRP-503 or an approved protocol template.

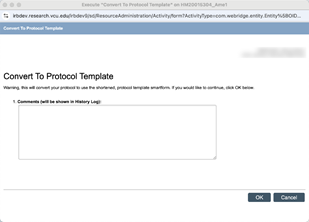
This may also be done after system go-live using the Create Modification/CR activity.
-
Alternatively, study teams may wait until the first study modification after go-live to transition to a protocol. Personnel-only modifications do not require transition to a protocol.
-
By the time of required continuing review, all studies must complete transition to a protocol template. Exceptions to this are listed below.
HRS IRB system transition
First touch options for active studies
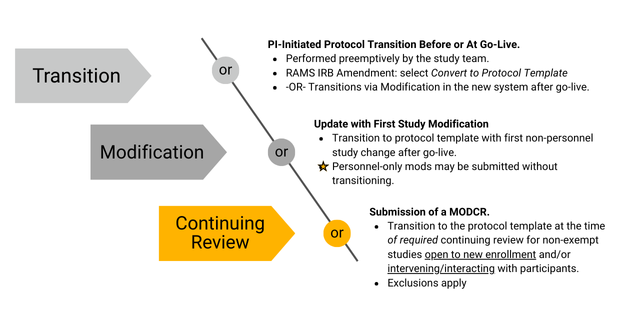
(Text description for image above)
Summary
Which studies do NOT need to transition to protocol based applications?
- Exempt studies
- Expedited studies that do not require continuing review
- Full board / greater than minimal risk studies that are active for data analysis only or are in long term follow-up
Which studies DO need to transition to a protocol?
- Any study, including studies eligible for expedited review, required to undergo at least annual continuing review, including FDA or DOJ-regulated research
- Full board / greater than minimal risk studies that are open to enrollment or where research interactions (apart from those qualifying as long-term follow-up) and interventions are ongoing
- Individual studies at the discretion of the IRB (e.g., studies not requiring continuing review but where changes continue to be made over time)
Please contact the HRPP with questions about whether your study is required to transition to a protocol in the coming months.
| Example RAMS IRB extended smartform fields (pre-Toolkit go-live 7/14/23): | Reduced RAMS IRB smartform effective 7/14/23: |
|---|---|
|
|
|
Our new system shares the same base platform as the current RAMS IRB system, meaning many features and workflows will be familiar to our research community. Enhancements to the PI Inbox and visualization of the review process, improved workflow reporting and data analytics capabilities and expanded RNI submission options are included in this updated system.
The new system will retain the ability to copy a submission, communicate with IRB staff and members to make changes to study materials or clarify submission content, maintain version history for IRB approved documents, and pull-down approval letters.
Visualize the review process
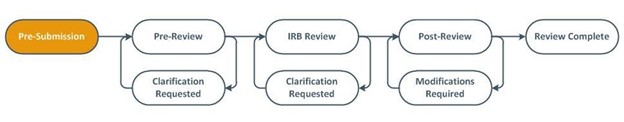
(Text description for image above)
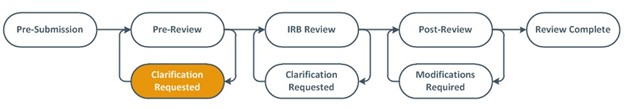
Study teams may observe their submission moving through the staff-driven administrative pre-review process, into designated or committee IRB review phase, into post-review where approval letters may be obtained. Submissions requiring PI-responses will be indicated by the clarification requested or modifications requested workflow state.
- Streamlined SmartForm with protocol-based application (HRP-503/a)
- Other VCU Requirements has been removed from the VIRBs SmartForm
- Reference HRP-309 - Ancillary Review Matrix for required institutional approvals
- Protocol Editors are now PI Proxies
- Must be listed as study personnel
- Included in conversion from RAMS to VIRBs for current studies
- Department head approval facilitated through new IRB system activity
- PI will initiate review via the Manage Ancillary Reviews activity
- Submission will be able to enter the IRB workflow but review will not commence before sign-off
- VIRBs now has an IRB Staff Pre-Review Clarifications Requested state
- Staff will communicate clarifications or changes needed to proceed with IRB review
- VIRBs does not have SmartForm Reviewer Notes
- New external IRB reliance workflow
- Approval to Rely on an External IRB is granted in the administrative Pre-Review state
- Study teams will upload the external IRB approval letter and final approved local study documents (e.g., ICF, recruitment material) in the Pending sIRB Review state

- External IRB reliance workload utilizes Study Updates for follow-on submissions related to CR, mods, updates or reportable new information

- sIRB reliance workflow is reflected differently in VIRBs
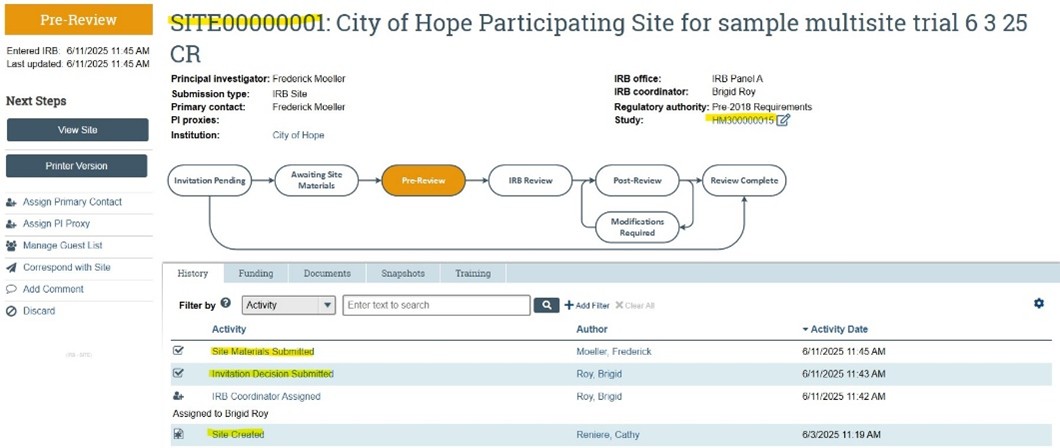
- Approval for VCU to serve as the IRB of record (sIRB) is reflected in the main study submission SmartForm
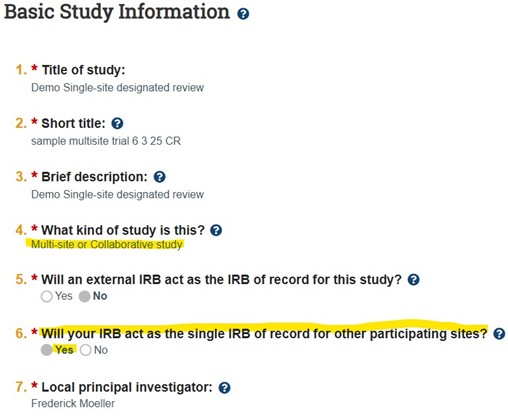
- Approval for VCU to serve as the IRB of record (sIRB) is reflected in the main study submission SmartForm
- Participating Sites (pSites) are added after initial study approval

Contact us
Please use the following contact information for:
- Reporting for single patient access and emergency uses of FDA regulated products, or
- General questions and questions related to IRB submissions

Questions related to single IRB, reliance agreements and/or external IRBs:

For individual staff email addresses, please see Contact us page for staff directory