The VCU IRB provides oversight to all activities that meet the regulatory definitions of "research involving a human subject," meaning that any activity meeting the definition of research, as well as the definition of human subject, requires IRB review.
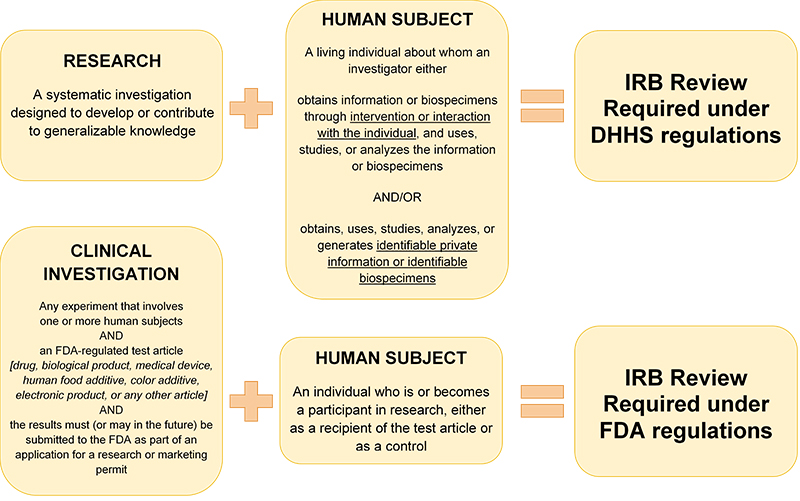
Research is a systematic investigation designed to develop or contribute to generalizable knowledge. Human subject research involves collecting information or biospecimens about living individuals.
Refer to HRP-310 - WORKSHEET - Human research determination to help you determine if your projects involves human subject research or how it is regulated.
Activities that are not research
Scholarly and journalistic activities
These activities include the collection and use of information that focus directly on the specific individuals about whom the information is collected (e.g., oral history, journalism, biography, literary criticism, legal research and historical scholarship). Please review HRP-320 - WORKSHEET - Scientific or scholarly review.
Further guidance can be found on OHRP’s website.
Public health surveillance activities
These activities must be conducted, supported, requested, ordered, required or authorized by a public health authority. They should be limited to only those necessary to allow a public health authority to identify, monitor, assess or investigate potential public health signals, onsets of disease outbreaks, or conditions of public health importance.
Further guidance can be found on OHRP’s website.
Examples of activities related to public health that WOULD meet the definition of research because the purposes, context or nature of these activities is to create generalizable knowledge:
- Epidemiological research
- Research evaluations of public health surveillance activities
- Subsequent research using information collected during a public health surveillance activity (e.g., genetic analysis of biospecimens)
- Exploratory studies designed to better understand risk factors for chronic diseases, including genetic predisposition for chronic diseases
- Exploratory studies designed to elucidate the relationships between biomarkers of exposure and biomarkers of disease
- Exploratory studies of potential relationships between behavioral factors (e.g., diet) and indicators of environmental exposures
Quality improvement activities
Most quality improvement efforts do not meet the definition of research because they are not designed to be generalizable.
The intent of a quality improvement activity is to improve a specific business practice. In a hospital, this may include improving the quality and/or consistency of care in a specific unit or the entire hospital.
Examples of other activities that generally do not meet the definition of research:
- Student projects conducted for a credit class assignment where there is no intent to contribute to knowledge in the field of study
- Medical case studies involving no more than two patients are not considered systematic investigations
- Program evaluation activities where the data will only be used internally and there is no intent to publish or present the outcome
Research only using data about deceased individuals
Investigators accessing or using protected health information about decedents for research purposes must submit a HIPAA Research on PHI of Decedents Certification Form.
Secondary data that are obtained by the investigator in a completely anonymous state when the investigator will have no access to the ability to re-identify individuals.
See the Research Involving Private Information or Biological Specimens decision chart.
Secondary research involving non-identifiable newborn screening blood spots is not considered research involving human participants.
The Newborn Screening Saves Lives Reauthorization Act of 2014 will no longer be effective following the effective date of the Revisions to the Common Rule, given that its changes applied only until changes to the Common Rule were promulgated.
The VCU IRB must approve all research involving human subjects where VCU is engaged. The university is engaged in research when one or more of the following apply:
- The human subjects research is sponsored by VCU
- VCU receives a direct federal award to conduct human subjects research, even when all activities involving human subjects are carried out by a subcontractor or collaborator
- The human subjects research is conducted, in whole or in part, by members of the VCU faculty, staff or students acting in their university capacity regardless of the location of the research
The VCU IRB refers to the HRP-311 - WORKSHEET - Engagement determination when determining whether a site is engaged in human subjects research.
The following clinical activities are not research, but FDA regulations require that they are reviewed by the IRB.
Non-research activities involving investigational products
Investigational drugs, biologics and devices are sometimes used for treatment of serious or life-threatening conditions either for a single subject or for a group of subjects.
Treatment using a humanitarian use device
A humanitarian use device is a medical device that has been given a special type of FDA marketing approval with certain profit and use restrictions because it has potential therapeutic benefit for a small population of patients but has not been proven effective.
The HRP-103 - Investigator manual includes specific instructions for reporting/submitting emergency uses of FDA regulated products, Humanitarian Use Device (HUD) protocols, and compassionate use of a device to the IRB.
For specific FDA regulations that must be considered during the IRB’s review, refer to Appendix A-2 Additional Requirements for FDA-Regulated Research in the Investigator Manual.
The IRB applies the HRP-322 - WORKSHEET - Emergency use, HRP-323 - WORKSHEET - Criteria for approval humanitarian use device (HUD) for approval humanitarian use device (HUD), and HRP-325 - WORKSHEET - Device compassionate use for specific regulatory guidance that the research team and IRB must address for the institution to move forward with specific uses that apply to the patient condition and access to products that would not otherwise be available for their immediate care.
The IRB refers to the HRP-306 - WORKSHEET - Drugs and biologics and HRP-307 - WORKSHEET - Devices for research with FDA regulated investigational products. Additional institutional level guidance for conduct of FDA regulated activities at VCU can be found on the Regulatory affairs page.
At VCU, investigators are responsible for determining whether an activity requires IRB review. However, investigators may submit their project to the IRB if they wish the IRB to make an official determination.
My project requires VCU IRB review.
(The project is or might be “research involving a human subject" and VCU is engaged.)
If you have determined that IRB review is required, the next steps are to assess the project’s level of risk and what type of review is appropriate, exempt, expedited or full board, and then to submit the study to the IRB electronically using VIRBs (VCU IRB system). The IRB’s review decision will be sent in a letter to the principal investigator.
I don’t know whether my project requires VCU IRB review.
If a determination cannot clearly be made, submit the project to the VCU IRB using VIRBs (VCU IRB system), and the IRB will make an official determination. If the activity is submitted to the IRB, the review decision will be sent in a letter to the principal investigator.
My project does not require VCU IRB review.
(The project is not “research involving a human subject" or it is “research involving a human subject,” but VCU is not engaged.)
The project does not need to be submitted to the IRB. When an individual makes the determination that an activity does not constitute human subject research, the VCU IRB recommends that it be documented in writing (e.g., write a memo or note to file that explains how you think the definitions of research and/or human subject are not met). Then, request an acknowledgment/counter-signature from your department chairperson (or designee) to document that they concurred with your rationale. These records should be retained with other activity records.
If an official determination letter from the IRB is required, submit the project to the VCU IRB using VIRBs (VCU IRB system), and the review decision will be sent in a letter to the principal investigator. For ‘Not human research’ and ‘non-engagement’ determinations, complete and upload the HRP-503b - TEMPLATE NHSR for the IRB’s review.
The IRB reviewers apply the HRP-310 - WORKSHEET - Human research determination and the HRP-311 - WORKSHEET - Engagement determination when making determinations of NHSR and non-engagement through the IRB’s review process.
Refer to a decision chart provided by the Office of Human Research Protections to help determine whether an activity requires IRB review in most situations:
- Chart 1: Is an activity research involving human subjects covered by 45 CFR part 46?
- NIH decision chart: Research involving private information or biological specimens
- ORHP investigator responsibilities FAQ
- OHRP quality improvement activities FAQ
- OHRP guidance on coded private information or specimens use in research, guidance (2008)
Contact us
Please use the following contact information for:
- Reporting for single patient access and emergency uses of FDA regulated products, or
- General questions and questions related to IRB submissions

Questions related to single IRB, reliance agreements and/or external IRBs:

For individual staff email addresses, please see Contact us page for staff directory