Compassionate, expanded access and emergency use of drugs/devices
Criteria for expanded access to treatment
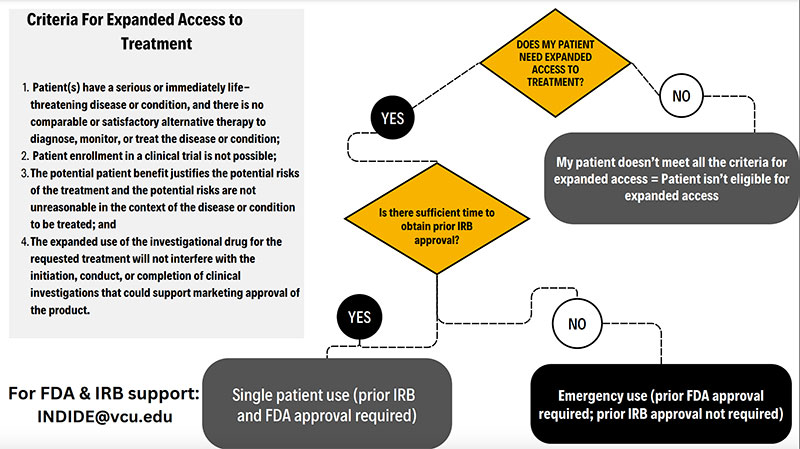 Decision tree - emergency use vs. single patient (text only version)
Decision tree - emergency use vs. single patient (text only version)
Compassionate use
Also known as compassionate use or a single patient IND, refers to the use of an investigational (not FDA approved) drug/device to diagnose, monitor or treat a patient or patients rather than obtain information that is normally collected in clinical trials. Those eligible for expanded access are patients with an immediately life-threatening disease/condition where the likelihood of death is within months or where premature death is likely without treatment or the condition/disease is substantially impacting daily functioning. Expanded access is used when there isn’t a comparable or satisfactory alternative and a clinical trial isn’t available. There are three sizes of compassionate use trials: single patient, intermediate-size and expanded access (treatment IND/ widespread use). This type of use is subject to FDA and IRB approval prior to initiating treatment. The VCU IRB requires IRB and FDA approval prior to initiating treatment.
Emergency use
This is used when there isn’t sufficient time to obtain FDA and IRB approval prior to starting treatment with an unapproved (investigational) drug/device. This is used when someone is in a life-threatening or seriously debilitating situation where there is no other treatment available and there is no time to obtain FDA and IRB approval. VCU requires the IRB to be notified via phone, email or fax prior to emergency use but the treating physician doesn’t need to wait for IRB approval. The treating physician must submit this to the VCU IRB within 5 business days.
FDA expanded access guidance and forms
- Form FDA 3926
- Individual patient expanded access applications: Form FDA 3926 guidance for industry
- Expanded access for medical devices
Compassionate/Emergency use templates
Additional resources
- Project Facilitate - Service provided by the FDA as a call and information center for oncology-related expanded access or single patient INDs
- Reagan-Udall Foundation (RUF) - An alternative submission process for single patient IND initial and follow up submissions. This website should not be used for emergency use IND requests. If the IND is submitted through RUF, it should not be submitted through the FDA normal submission channels (ex: CDER NextGen Portal).