Services
Jump to:
- Magnetic resonance imaging and spectroscopy
- Photoacoustic imaging
- PET imaging
- CT imaging
- Optical imaging
Magnetic resonance imaging and spectroscopy
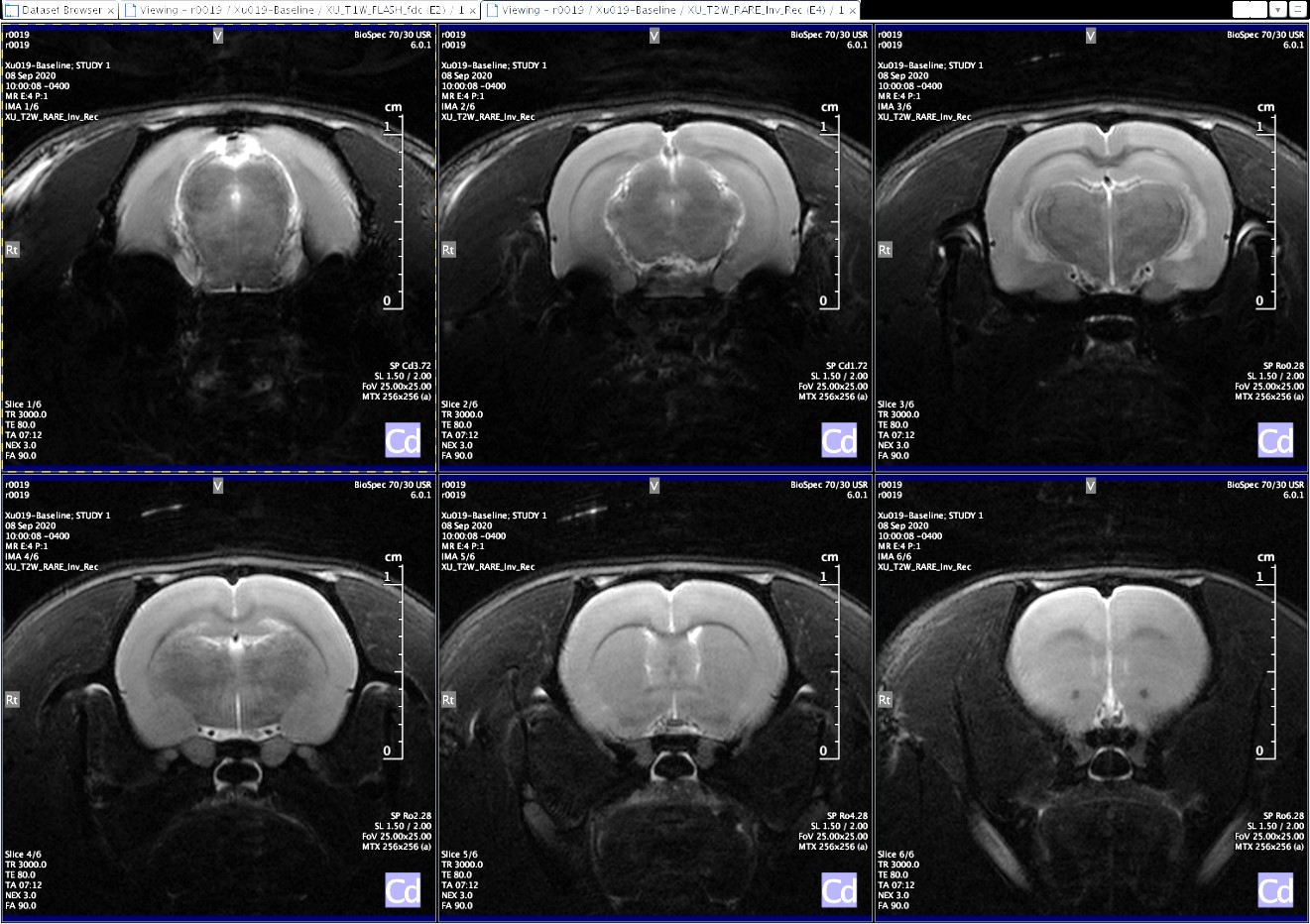
Magnetic resonance imaging is a high-resolution imaging modality capable of superior soft-tissue contrast. Magnetic resonance spectroscopy may be employed for assessment of tissue and organ function.
The Bioimaging and Applied Research Core maintains a 7 Tesla Bruker Biospec preclinical MRI scanner, capable of rodent and small mammal imaging with a broad range of image and spectroscopic acquisition paradigms. Examples of imaging and spectroscopic protocols include: T1- and T2-weighted imaging, diffusion weighted and diffusion tensor imaging, echo-planar imaging, ultra-short TE imaging, cardiac CINE imaging, and Point-resolved and Stimulated echo spectroscopic acquisition methods for proton and various X-nuclei.
Full physiological monitoring, including respiratory/cardiac gating and thermoregulation capabilities are available. In addition to proton imaging and spectroscopy, RF coils are available for fluorine, phosphorus and carbon acquisitions. MRI technical expertise is provided to assist researchers with optimization of imaging paradigms, data acquisition, and analysis.
Photoacoustic imaging
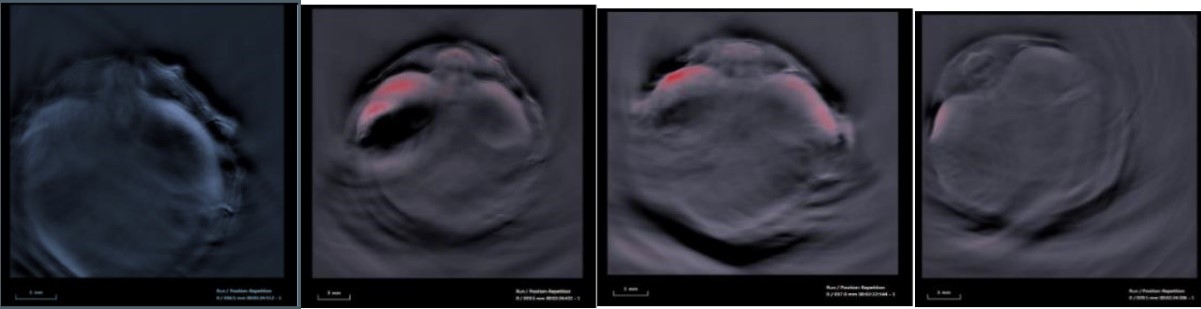
Photoacoustic imaging (PAI) is a hybrid modality that merges optical illumination with ultrasound detection to achieve high resolution optical contrast images to a few centimeters in depth.
PAI capitalizes on the intrinsic optical absorption of chromophores in tissue such as hemoglobin, melanin, and lipids. As each of these chromophores exhibits its own characteristic absorption spectra, PAI at multiple wavelengths allows for their relative quantification and helps to investigate physiological changes in disorders to understand the mechanism behind them and how they can be managed effectively.
The Bioimaging and Applied Research Core maintains an iTheraMedical MSOT inVision optoacoustic imaging platform for preclinical research.
PET imaging
Positron emission tomography (PET) cameras are capable of detecting paired 511 keV photons generated from the annihilation event of a positron and electron. The paired photons travel in opposite directions (1800 apart) along a line. These are detected by pairs of detectors kept on opposite sides in a ring configuration within the PET camera. This is called annihilation coincidence detection. Following the acquisition of many images of the positron emission, the data are reconstructed into a final static image or time series. PET has greater sensitivity and resolution combined with better quantitative abilities compared to other radionuclide detection modalities such as SPECT. See below for a number of vendor-supplied PET tracers that could become available for molecular imaging at BARC.
PET tracers available for molecular imaging from vendors provided through BARC
- [18F]-FDG - [18F]Fluoro-2-deoxy-2-d-glucose
- [18F]-FDG, a glucose mimetic, is the gold standard to measure alterations in glucose uptake in vivo, such as in brain, cancer, cardiovascular diseases, Alzheimer’s disease and other central nervous system disorders, infectious, autoimmune, and inflammatory diseases.
- [18F]-FLT - 3'-deoxy-3'-[18F]fluorothymidine
Uptake and accumulation of FLT are an index of cellular proliferation. [18F]FLT PET has been used to detect and monitor tumor growth, staging, and to detect metastases. - [18F]-NaF - sodium fluoride
(18F-NaF) PET/CT provides high sensitivity and specificity for the assessment of bone and joint diseases. Its binding accurately differentiates malignant from benign bone lesions, especially under dynamic quantitative approaches. 18F-NaF has also shown promising results for imaging artherosclerosis.
Efforts are underway to provide access to additional molecular imaging PET tracers to be obtained through vendors. BARC staff will help core facility users in selecting the appropriate tracer and scanning methodology for their research applications.
- [18F]-Fluciclovine (18F-Axumin; recurrent prostate cancer)
- [18F]-Fluoroestradiol (metastatic breast cancer marker)
- [18F]-Florbetaben (Alzheimer’s disease/β-Amyloid)
- [18F]-FAraG (T-cell tumor filtration and general immunomodulation)
- [18F]-FMISO (hypoxia and Tx resistance)
- [18F]-FHBG (PET reporter gene imaging)
- [89Zr]-CD8 (CD8 T-cell tumor infiltration)
CT imaging
CT scanning uses a motorized X-ray source that rotates around a circular opening called a gantry. During a CT scan, the subject is positioned on a bed within the gantry by defining a field of view (FOV). The X-ray tube rotates around the gantry, shooting narrow beams of X-rays through the body the subject. Instead of film, CT scanners use special digital X-ray detectors located directly opposite the X-ray source. As the X-rays pass through the subject, they are picked up by the detectors and transmitted to a computer. Each time the X-ray source completes one full rotation, the CT computer uses sophisticated algorithms to construct a 2D image slice of the patient. The thickness of the tissue represented in each image slice can vary depending on the CT machine used but usually ranges from 1-10 millimeters. When a full slice is completed, the image is stored and the motorized bed is moved forward incrementally into the gantry. The X-ray scanning process is then repeated until the desired number of slices is collected. Image slices can either be displayed individually or stacked together by the computer to generate a 3D image of the subject showing the skeleton, organs, and tissues with contrast based on their densities. These are quantified and expressed as Hounsfield units (HU), where a large negative value is assigned to air and 0 is assigned to water. This modality has many benefits such as the ability to rotate the 3D image in space or to view slices in succession, making it easier to find and study the exact regions of interest. Bones exhibit the highest density, while lung parenchyma and body fat have densities relatively lower than water in terms of HU normalization. The BARC maintains a
MultiScan LFER large bore research PET/CTfor CT scanning and can be used in tandem with PET acquisitions.
Optical imaging
Optical imaging uses light to interrogate cellular and molecular function in the living body, as well as in plant and in vitro samples. Images are generated by using photons of light in the wavelength range, from ultraviolet through near infrared. Contrast is derived through the use of:
- exogenous agents (e.g., dyes or probes) that provide a signal
- endogenous molecules with optical signatures (e.g., NADH, hemoglobin, collagen)
- reporter genes
Fluorescence protein imaging uses endogenous or exogenous molecules or materials that emit light when activated by an external light source such as a laser. An external light of an appropriate wavelength is used to excite a target molecule, which then fluoresces by releasing longer-wavelength, lower-energy light. Fluorescence imaging provides the ability to localize and measure gene expression including normally expressed and aberrant genes, proteins and other pathophysiologic processes. Other potential uses include cell trafficking, tagging superficial structures, detecting lesions, and monitoring tumor growth and response to therapy. The Bioimaging and Applied Research Core maintains a CRi Maestro2 multispectral fluorescence imaging system capable of in vitro and in vivo small mammal imaging with multispectral analysis.